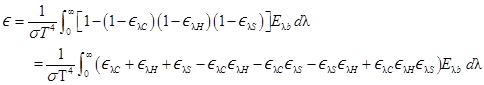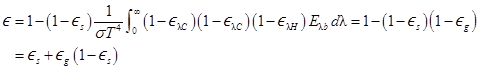|
UNIFORM SOOT SUSPENSION IN AN ISOTHERMAL RADIATING GAS
Usually, for soot in a flame or in combustion products, there
are gaseous constituents that also radiate energy. To simplify the present
discussion, it is assumed that only three radiating constituents are
present—carbon dioxide, water vapor, and soot—but the method can be extended to
more constituents. As spectral radiation passes through the gas-soot suspension,
the local attenuation depends on the sum of the absorption coefficients so, from
(9-65), the spectral emittance is
where the subscripts C, H, and S refer
to CO2, H2O, and soot. Then, from (D-12), the total
emittance is
With
The first three terms in the last integral yield the total
emittances of the three constituents, so that
A term such as
Results for the spectral-overlap terms were calculated in
Felske and Tien (1973, 1975) by using the form of the CO2 and H2O
vapor absorption bands and the soot absorption coefficient
kλ
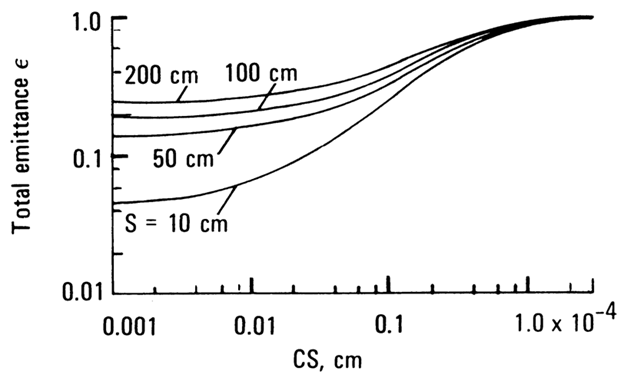
= Ck/λ discussed earlier. Typical emittances are in Fig. D-9. For
low values of CS the soot concentration is low, especially when S
is high. Hence the left sides of the curves are dominated by the gas emittance,
and the vertical displacement of the curves shows the increase in gas emittance
with path length. As CS is increased from 0.001, the curves are somewhat
horizontal (especially at high S, which corresponds to low Q as
the soot concentration is not sufficient to increase ε for the mixture
significantly. For larger CS the soot begins to dominate, and for all the
path lengths shown, ε approaches 1 when CS is about 3 × 10−4
cm. This is consistent with the results in Fig. D-6.
Since soot has a rather continuous emission spectrum, it is
reasonable [Felske and Charamapoulos (1982)] to assume gray emission of soot in
a nongray gas. This leads to a particularly simple form relating the emittance
of the suspension to the individual emittances of the soot and gas. If there is
no soot, Eq. (D-27) becomes
Then, from (D-27), if the soot is gray,
The sum of gray gases model [see Eq. (9-54)] was used in
Felske and Charamapoulos to represent the emittance for a mixture of soot,
CO2, and H2O vapor,
where bj
is a function of the soot volume fraction and the partial pressures of the CO2
and H2O. This model was used in Smith et al. (1987) to compute
radiative transfer for a gas-soot mixture between parallel plates. Kunitomo
(1974) gives results for the ratio, in a flame, of the soot-cloud emittance to
the emittance of the nonluminous suspending gas; these results are for a liquid
fuel. The ratio increases as the fuel carbon-hydrogen ratio is increased and as
the excess air is decreased. Babikian et al. (1990) made mass absorption
coefficient measurements of soot in spray combustor flames. Values were found to
be between those in Dalzell and Sarofim (1969) and in Lee and Tien (1981). |