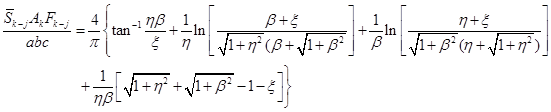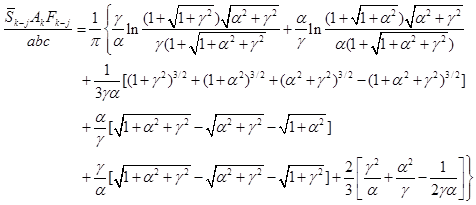|
A.7 Geometric-Mean Beam Length for Spectral Band Enclosure
Equations
For use in the spectral band enclosure equations (10-82), the
absorption integral in (10-85) must be evaluated between pairs of enclosure
surfaces for the wavelength bands involved. When more than a few bands absorb
appreciably, the enclosure solution requires considerable computational effort.
A simplification was developed by Dunkle (1964) by assuming that the integrated
band absorption
where Sc and δ are the line
intensity and the spacing of the individual weak spectral lines, as in Chap. 9.
Now define a mean path length
which depends only on geometry. This integral is also
obtained in Eq. (A-1) when kλS
is small (optically thin limit). In Dunkle, Sk–j values are
tabulated for parallel equal rectangles, for rectangles at right angles, and for
a differential sphere and a rectangle. Analytical relations for rectangles are
in Eqs. (A-16a,b). For directly opposed parallel equal rectangles
with sides of length a and b and spaced a distance c apart,
(A-16a)
where η = a/c, β = b/c and
where α = a/b and γ = c/b. The Fk–j
is obtained from Factor 8 in book Appendix C. Results for equal opposed parallel
rectangles are in Fig. A-8, and values for equal parallel rectangles and for
rectangles at right angles are in Tables A-1 and A-2. Other
For a medium at uniform conditions, the geometric-mean beam
length can be used in the effective-bandwidth correlations in Chap. 9 to obtain
The wavelength span Δλl of each band is
needed to carry out the solution. As discussed after (9-24), this span can
increase with path length. Edwards and coworkers [Edwards and Nelson (1962),
Edwards (1962), Edwards et al. (1967)] give recommended spans for CO2
and H2O vapor; these values, in wave number units, are in Table A-3
for the parallel-plate geometry. For other geometries, Edwards and Nelson give
methods for choosing approximate spans for CO2 and H2O
bands. Briefly, the method is to use approximate band spans based on the longest
important mass path length in the geometry being studied. With this in mind, the
limits of Table A-3 are probably adequate for problems involving CO2
and H2O vapor. |